The hydrologic system of the UIR watershed, with its shallow to bedrock soils, active karst features, and surface to groundwater interactions, intensifies water quality issues. The Iowa DNR draws attention to the vulnerability of surface water and groundwater in karst terrain like that found in the UIR Watershed on their webpage, “Karst Terrain and Sinkholes” They note the following.
“Karst terrain is characterized by the presence of easily dissolved bedrock (limestone and dolomite) near the ground surface. Because carbonate rocks can be dissolved by groundwater, karst areas are often characterized by sinkholes, springs, and losing streams where some surface flow is lost to groundwater. Groundwaters and surface waters in these areas are highly vulnerable to contamination because contaminants can travel quickly from the surface through open fractures and caves to aquifers, springs, and streams and are not likely to be filtered by soils.”
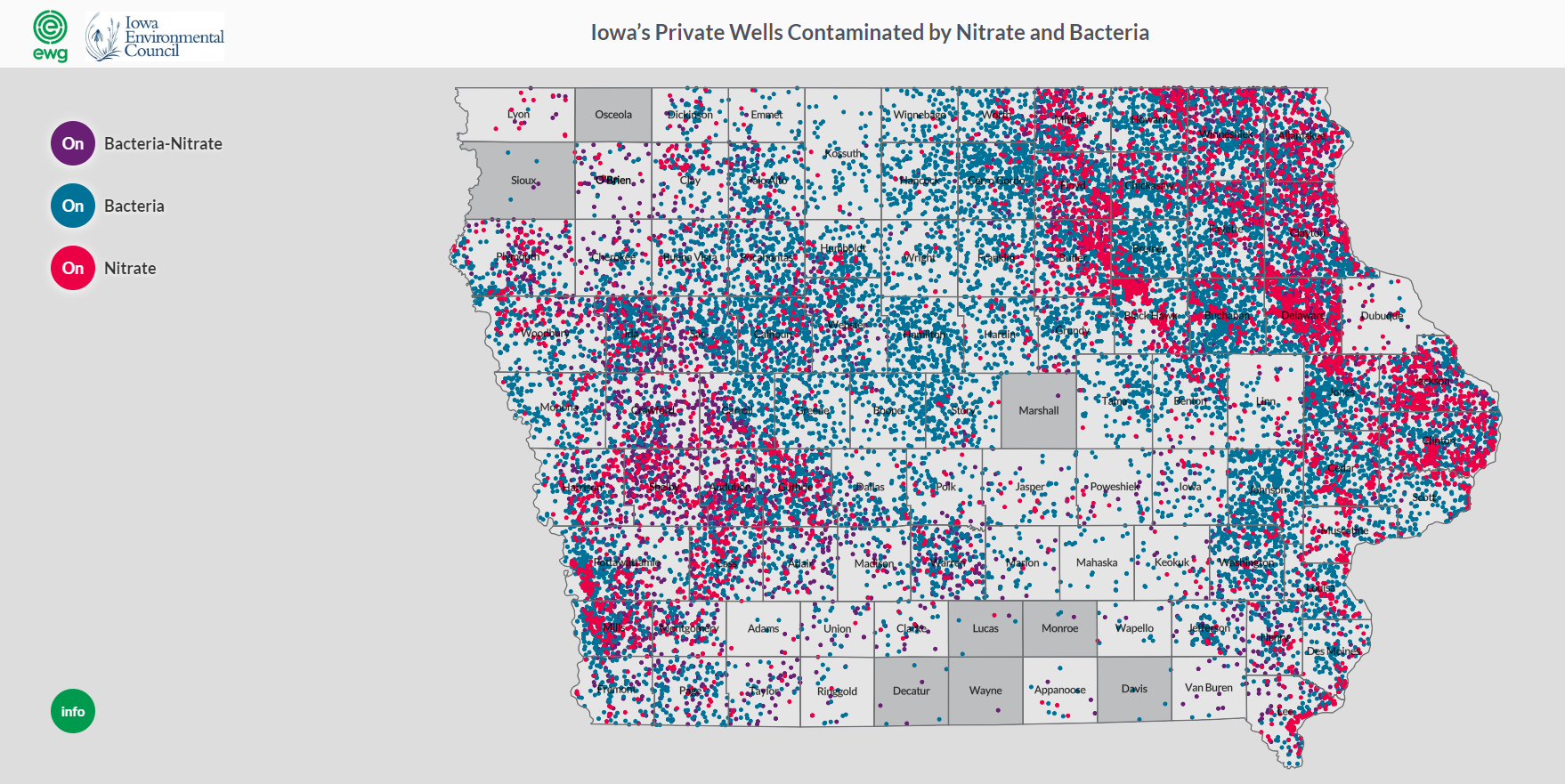
The interactions between surface and groundwater and the vulnerability of groundwater is also demonstrated in a survey of state well test results from thousands of private wells in Iowa. The data came from Private Well Tracking System organized by the Iowa DNR through a public records request. The survey, conducted by the Environmental Working Group and Iowa Environmental Council found that nitrate, coliform bacteria and fecal coliform bacteria were confirmed in thousands of wells across Iowa between 2002 and 2017. The study notes that both contaminants have serious impacts on public health and that not all wells are tested. More information about this study can be found here. A map developed through the research shows that the Upper Iowa River Watershed is located in an area of Iowa that has documented significant numbers of contaminated wells.
Geology’s Affect on Groundwater Flow
Similar to Northeast Iowa and the Upper Iowa River Watershed, Southeast Minnesota contains geologic features that affect the movement of water from the surface through layers of soil and rock below. The Minnesota Department of Agriculture along with many partners developed a video series that illustrates the unique geologic features, movement of water to bedrock aquifers and streams, and the vulnerability to contaminates like nitrate in the root river watershed in southeast Minnesota. These videos create a great visual explanation of how groundwater moves throughout the Driftless Area, which includes the Upper Iowa River Watershed. Check out all the videos and illustrations at https://www.mda.state.mn.us/segwresources . Watch the video below to learn about how contaminates like nitrate move through the unique bedrock geologic features in the Driftless Area.
History of Water Sampling
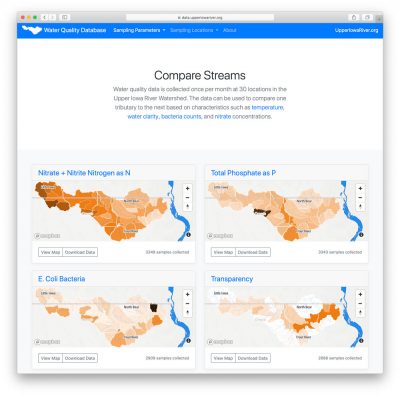
For the past 19 years, Northeast Iowa RC&D has coordinated partners and raised funds to implement water quality monitoring in the Upper Iowa River Watershed. The partners have teamed up to collect a remarkable amount of water quality data from 30 locations across the watershed. Since 2004, samples have been taken once a month, from April through October, at 3 locations along the main channel of the Upper Iowa River and 27 locations near the mouth of major tributaries. This data provides a snapshot of the entire watershed on a given day each month (e.g., 2nd Tuesday) and allows the comparison of one stream/watershed to the next. Water quality data for the Upper Iowa River Watershed can be found on the following website https://data.upperiowariver.org. For example, watersheds of streams in Howard county can then be compared and contrasted with similarly-sized watersheds that empty into the Upper Iowa 100-miles downstream in Allamakee county. Water samples are collected on the same day by five public and private organizations, packed on ice, and sent to a lab for analysis. From 1999–2013, samples were analyzed by the State Hygienic Laboratory, and beginning in 2014, by the laboratory at Coe College run by Marty St. Clair. The samples are analyzed for concentrations of E. coli Bacteria, Nitrate and Nitrite Nitrogen as N, Total Phosphate as P, Chloride, Sulfate, Total Suspended Solids, and in past years: Ammonia Nitrogen as N, Atrazine, and Fecal Coliform. Field measurements are also taken, including temperature, pH and water transparency.
Extensive data collection over a wide spatial area of the UIR Watershed has allowed for a better understanding of the water quality problems in the watershed, and has allowed landowners, citizens, and organizations to find ways to solve the issues related to poor water quality. It has resulted in increased funding for effective subwatershed projects, bringing in tens of millions of dollars in state and federal cost share to help private landowners implement voluntary conservation practices, as well as funding for SWCDs and other organizations to hire conservation professionals, including technicians and engineers, to provide free technical assistance to watershed residents.
The local monitoring effort, the expertise and dedication of a diverse group of private and public partners, and the quality of the resources have convinced state and federal agencies and nonprofit organizations to invest more dollars for voluntary conservation in the UIR Watershed over the past 15 years than almost any other HUC 8 watershed in Iowa.
Water Quality Parameters
Nitrogen
Nitrogen is an essential plant nutrient, but excess nitrogen can cause water quality problems. Too much nitrogen and phosphorus in surface waters causes nutrient enrichment, increasing aquatic plant growth and changing the types of plants and animals that live in a waterbody. This process, called eutrophication, can also affect other water quality parameters such as pH and dissolved oxygen. Nitrate and nitrite are two forms of nitrogen. Nitrate is very easily dissolved in water and is more common in streams. Sources of nitrate include soil organic matter, animal waste, decomposing plants, sewage, and fertilizers. Because nitrate is very soluble in water it can move readily into streams. Nitrite is another form of nitrogen that is less common because it is quickly converted to nitrate or returned back to the atmosphere as nitrogen gas. Due to its instability, detectable levels of nitrite in streams and lakes are uncommon. Detectable nitrite levels in streams and lakes may indicate a relatively fresh source of ammonia. The amount of nitrate or nitrite dissolved in water is reported as Nitrate-N (nitrate expressed as the element nitrogen) or Nitrite-N in milligrams per liter of water (mg/L). Iowa’s drinking water standard for nitrate is 10 mg/L as Nitrate-N. The concentration of Nitrate-N in water may vary greatly depending on season and rainfall, fertilizer application rates, tillage methods, land use practices, soil types, and drainage systems. Consistently high nitrate readings (over 10 mg/L) is a great concern and further investigation should be conducted
Phosphorus
Phosphorus is an essential nutrient for plants and animals and is usually present in natural waters attached to sediment and organic material. Most elevated levels of phosphorus are caused by human activities, including human, animal, and industrial waste, as well as runoff from fertilized cropland and lawns. Excess phosphorus in water speeds up plant growth, causes algal blooms, and can result in low dissolved oxygen (hypoxic conditions), that can lead to the death of certain fish, invertebrates, and other aquatic life. The amount of phosphate dissolved in water is expressed in milligrams per liter of water (mg/L).
Water Temperature
Many of the chemical, physical, and biological characteristics of a stream are directly affected by water temperature. Some species, such as trout, are quite sensitive to temperature changes. Water temperatures can fluctuate seasonally, daily, and even hourly. Thermal pollution can be caused by releasing warmed water into a stream from industry discharges or runoff from paved surfaces, removing riparian corridors, and soil erosion. Increases in water temperature can decrease the amount of oxygen dissolved in water, as cooler water holds more oxygen.
Oxygen
Certain processes add oxygen to a waterbody, while others remove or consume oxygen. Oxygen is added to a stream or lake from the atmosphere through mixing in turbulent areas. Plants also contribute oxygen through photosynthesis. Oxygen is removed in surface water by decomposition of organic material, respiration, and chemical processes. Increases in temperature can also increase the sensitivity of organisms to disease, parasites, and toxic wastes. Human impacts are most critical during the summer, when low flows and higher temperatures can cause greater stress on aquatic life.
Transparency
Transparency is a measure of water clarity and is affected by the amount of material suspended in water. As more material is suspended, less light can pass through, making it less transparent. Suspended materials may include soil, algae, plankton, and microbes. Transparency is measured using a transparency tube and is measured in centimeters. Low transparency indirectly harms aquatic species when solids settle out and clog gills, destroy habitat, and reduce the availability of food. Furthermore, suspended materials in streams promote solar heating, which can increase water temperatures, and reduce light penetration, which reduces photosynthesis, both of which contribute to lower dissolved oxygen. Sediment also can carry chemicals attached to the particles, which can have harmful environmental effects. Sources of suspended particles include soil erosion, waste discharge, urban runoff, eroding stream banks, disturbance of bottom sediments by bottom-feeding fish (e.g., carp), and excess algal growth.
pH
pH is a measure of a water’s acid/base content and is measured in pH units on a scale of zero to 14. A pH of seven is neutral (distilled water), while a pH greater than seven is basic/alkaline and a pH less than seven is acidic. The pH level of surface water is influenced by the concentration of acids in rain and the types of soils and bedrock in an area. Low pH levels (acidic) can have a harmful impact on the health of aquatic communities. The pH of Iowa surface waters generally ranges from 8.0 to 8.4. The presence of alkaline (basic) soils and limestone bedrock in many areas of the state help neutralize the effect acidic precipitation might have on Iowa’s streams and lakes. This is quite fortunate for Iowa since pH can influence many chemical and biological processes. Most aquatic organisms require habitats with a pH of 6.5 to 9.0.
Chloride
Chloride is a chemical element found in salts, which tend to dissolve easily in water. In natural waters, elevated levels of chloride may indicate inputs of human or animal waste, or inputs from fertilizers, many of which contain salts. During winter months, elevated chloride levels in streams may occur as a result of road salt runoff to nearby streams. The amount of chloride dissolved in water is expressed in milligrams per liter of water (mg/L). Average chloride concentrations for Iowa streams range from 16 to 29 mg/L.
E. coli Bacteria
E. coli bacteria is a fecal indicator bacteria that is used to detect pathogens in water. If ingested, pathogens in streams or rivers can cause human health illness. The primary source for most bacterial pollution in streams is from contact with animal and human waste, through agricultural activities or human wastewater systems. Over half of the HUC 12s monitored in the UIR Watershed average E. coli concentrations above 235CFU/100ml E. coli, this value represents Iowa’s single sample maximum water quality standard for primary contact recreational use. E. coli bacteria as an indicator species is a great concern for the UIR watershed. Many of its waters are unsafe for recreational use and human consumption.